
Serialization Is and Remains at the Top of the Priority List
Drug serialization from Asia to South America
The regulatory process for drug serialization has been completed in Bahrain, Uzbekistan, and the United Arab Emirates. Other health ministries are now planning how to protect consumers with drug serialization laws effectively.
With the global fight against counterfeit drugs, drug serialization requirements have become important for traceability and verification. This is important to protect consumers and manufacturers from counterfeit medicines effectively. In Germany, France, and other EU countries, corresponding solutions are already being used successfully. Other countries, such as Bahrain and Uzbekistan, have caught up in recent years and introduced mandatory tracking and serialization. You learned more about this in our first blog article. But what about the rest of the world? - We give you an insight.
Global Grug Serialization on the Fly
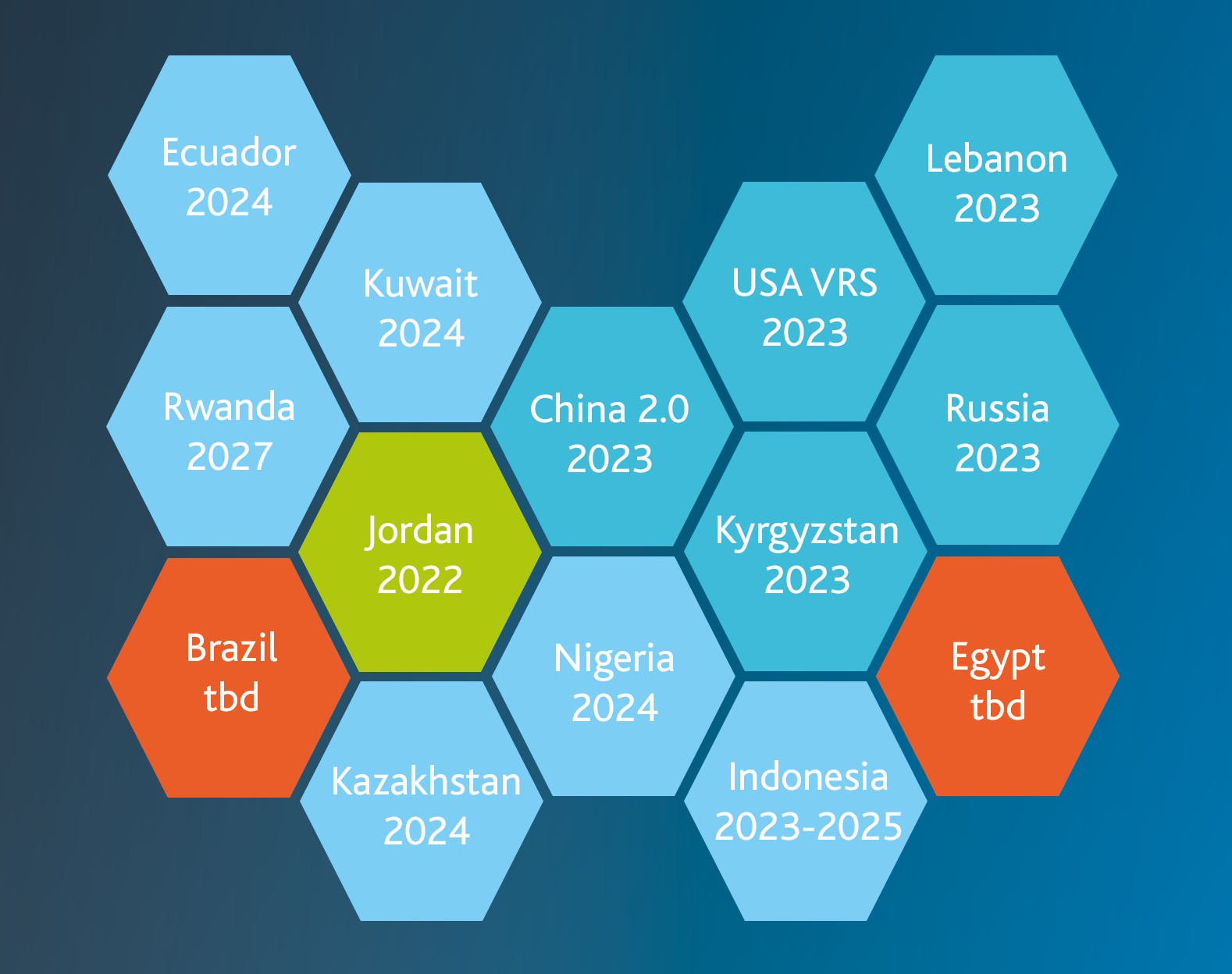
Lebanon: The deadline for drug serialization was reached recently, in January 2023, making the implementation and application of a data matrix code on all packaging mandatory by law.
Egypt: In the North African country, drug reporting already began in 2018. A new decision on a detailed regulation has yet to be made.
Indonesia: A new regulation was published only recently. However, further information on a detailed schedule has yet to be available.
Ecuador: The deadline for "phase 1" medicines expired in November 2022. Since then, traceability in this phase has extended to 400 products in the public health system (RPIS).
Russia: Currently, the API version of the OMS System 3.0 is supported on both demo and production tracks. Support for both versions is tentatively planned until the end of 2023. Support for OMS System version 2.0 is expected to end at that time.
Kuwait: In Kuwait, the deadline for drug serialization and tracking has been set for January 2024. Thus, all local requirements must be implemented by the end of 2023.
Rwanda: For Rwanda, the timetable is still tight enough for many other countries. Gradual serialization and aggregation are scheduled to take place by 2027.
Nigeria: As an emerging country, the African state is also heavily affected by counterfeit medicines. The gradual introduction of serialization is intended to counteract the trade in counterfeit medicines and is to be implemented by 2024.
Jordan: Serialization has already been introduced in Jordan. There are currently no plans to switch to mandatory tracking. However, the Jordanian Food and Drug Authority has issued a new circular setting a deadline of June 30, 2023, for the application of data matrix codes and serialization on secondary packaging of pharmaceutical products.
Kyrgyzstan: Local authorities have published the decree of the Minister of Health #1110 on the serialization and traceability of medicines in Kyrgyzstan. This indicates that the voluntary wave of aggregation will begin in January 2023, and the exact timelines are still under discussion. In detail, the following points are already regulatory:
Those who wish to be users of the system must have a valid Kyrgyz license and a permanent local establishment
- Responsibility for completing serialization rests with the importer
- Coding requirements: GS1 data matrix including GTIN + SN + ASCII separator.
- Batch expiration date and aggregation are optional
- Products carrying a Crypto Code from other countries are accepted
- Product must be serialized before certification (stickers are acceptable)
Other products that prospectively fall within the scope have yet to be defined. A voluntary list is to be drawn up by the Medicines Agency.
China: On December 31, 2020, the deadline for mandatory traceability of critical pharmaceuticals expired. Since then, two coding systems have been issued in China: One is the GS1 standard, which uses a GTIN as the product code as well as being encoded with a data matrix, and the other is the AliHealth eCode, which consists of a 20-digit code as well as a barcode.
New standards will come into force in 2023, announced the Chinese National Medical Products Administration (NMPA). The exact implementation schedule for all products has yet to be clarified. Other changes have already been agreed upon and communicated. These include requirements for the placement and print quality of the codes and placement on the sales unit rather than just the shipper unit or pallet. There will be no changes to the information that must be reported to the verification system.
USA: Back on November 27, 2013, the Drug Quality and Security Act (DQSA) was passed by Congress. Title II of the DQSA, the Drug Supply Chain Security Act (DSCSA), describes the steps necessary to achieve interoperable and electronic tracking of products at the package level. This is intended to identify and track certain prescription drugs as they are distributed in the United States. Part of these requirements mandates by law that product verification is necessary for certain circumstances. The phased approach will reach its final phase on November 27, 2023. As the deadline approaches, the growing use and experience of the Verification Routing Service (VRS), which uses the Lightweight Verification Messaging Standard (LVMS), has identified additional requirements beyond saleable returns. As a result, the pharmaceutical industry expanded its use of verification to include investigation of suspicious or illegitimate products, exception processing, and status checks.
Arvato Systems and the author cannot guarantee the accuracy of the information given, as regulations and deadlines change daily. If you have any questions, please do not hesitate to contact the team directly.
Written by






